Events
Katharina Pallitsch
University of Vienna, Austria
Deoxyfluorinated rare sugars to study the pentose phosphate pathway
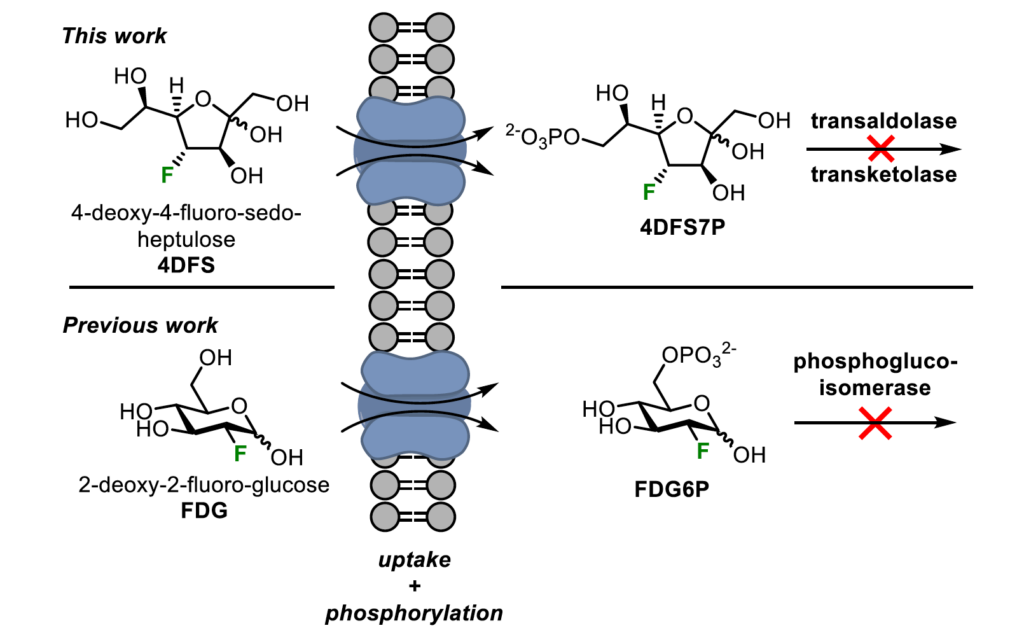
Deoxyfluorinated sugars are important tools to study carbohydrate metabolic pathways. Fluorinating specific positions within the sugar scaffold can lead to an enhanced metabolic stability compared to the native compound and subsequent metabolic trapping in cells. This rationale led to the development of the most widely used positron emission tomography (PET) tracer in clinics around the world: 2-deoxy-2-fluoroglucose (FDG).1 Despite this success story, the principle was never applied to study the pentose phosphate pathway (PPP) as the involved rare sugar phosphates were thought to be insignificant metabolic intermediates. The recent discovery of specialized kinases for D-ribulose, D-xylulose and D-sedoheptulose however raises questions on the extent to which cells consume and rely on these rare sugars in health and disease.
Thus, we designed and synthesised a set of deoxyfluorinated analogues of D-sedoheptulose,2 D-ribulose and D-xylulose,3 as well as of precursors for the future synthesis of their radiolabelled counterparts. We studied their uptake and phosphorylation by human fibroblasts and could show the phosphate of 4-deoxy-4-fluoro-sedoheptulose (4DFS) to effectively halt its enzymatic degradation by transaldolase and transketolase.
References:
1- E. Campbell, C: Jordan, R. Gilmour, Chem. Soc. Rev., 2023, 52, 3599-3626
2- L. Scheibelberger, T. Stankovic, M. Pühringer, H. Kählig, T. Balber, E. Patronas, E. Rampler, M. Mitterhauser, A. Haschemi, K. Pallitsch, Chem. Eur. J. 2023, e202302277.
3- L. Scheibelberger, T. Stankovic, K. Liepert, A. Kienzle, E. Patronas, T. Balber, M. Mitterhauser, A. Haschemi, K. Pallitsch, Eur. J. Org. Chem. 2023, 26(31), e202300339.
—
Katharina Pallitsch,a,* Toda Stanković,a Lukas Scheibelberger,a,b Kaja Lieperta
aInstitute of Organic Chemistry, University of Vienna, Währingerstraße 38, 1090 Vienna, Austria
bVienna Doctoral School in Chemistry (DoSChem), Währingerstr. 42, 1090 Vienna, Austria.